Applied thermodynamics is of fundamental importance in process design and for a wide range of applications in many industries. Indeed, the thermodynamic modelling activity is at the heart of the development and optimization of industrial processes.
GASP competencies in this field cover the study of phase diagrams – a preliminary but key research activity to determine suitable operating conditions for a process and to avoid undesired malfunctioning – and the selection and proper calibration of thermodynamic models for pure compounds as well as for mixtures. The goal of this latter research activity is to investigate the capabilities of existing models in providing a reliable representation of the system of interest and to develop new ones for novel specific applications. The model validation is performed by comparison of calculation results with the experimental data available in the open literature or with those collected at the Process Thermodynamics laboratory (PT lab). Different thermodynamic models are considered to deal with different types of systems (also including electrolyte systems) and phase equilibria (involving not just fluid phases but also solid species (e.g., solid CO2) and/or gas hydrates).
By developing adequate thermodynamic/kinetic/mass transfer models according to a multiphysic approach, ad hoc subroutines have been also developed and linked to commercial simulation software packages.
This research activity is closely related to the GASP strategic areas of CCUS (thermodynamics of CO2 capture, utilization and storage), natural gas processing, hydrogen economy and downstream separations in bio-processes.
a) 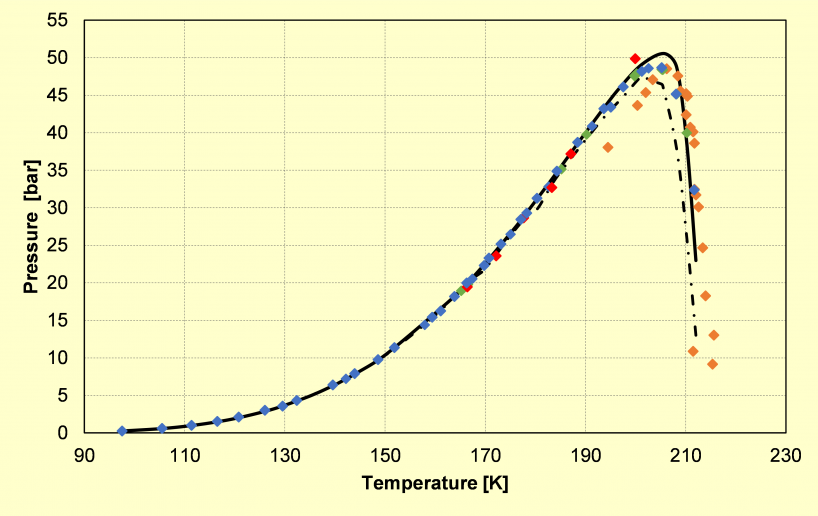
b) 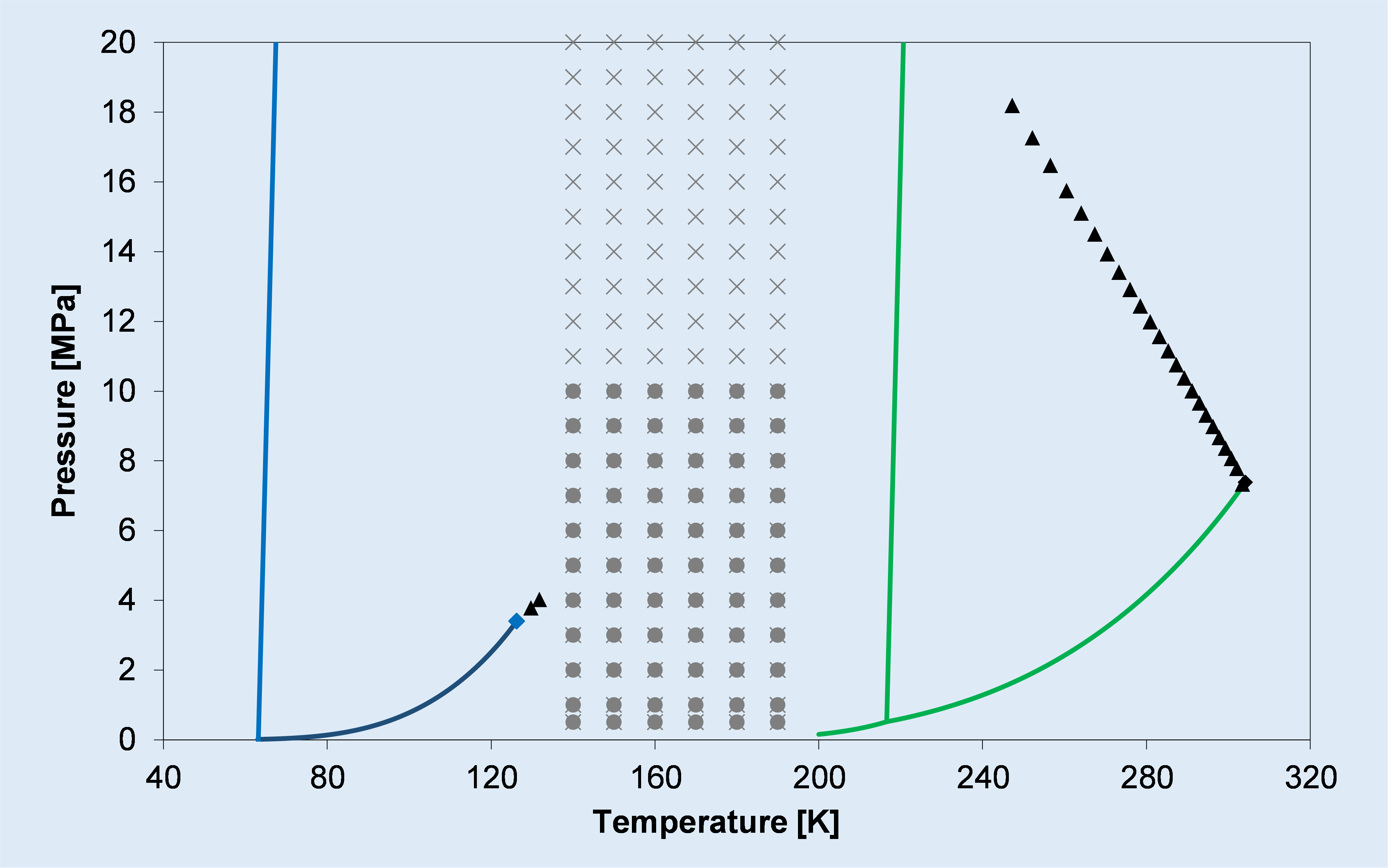
Phase diagram of: a) the binary CO2-CH4 system (type I), showing its calculated and experimental solid-liquid-vapor equilibrium locus; b) the binary CO2-N2 system (type III), showing its calculated critical locus (triangles) and solid-vapor equilibrium data (grey symbols) taken from the literature.
Back to Research.